


 Français
Français


Necrotic enteritis (NE) has a major impact on the broiler industry globally, leading to reduced gain, higher feed conversion, poorer health and welfare in clinical cases, and significant economic losses due to subclinical cases of the disease.
There have been decades of research taking place around the world in search of a vaccine against NE (including by the Canadian Poultry Research Council). A new vaccine produced by Huvepharma®, Clostridium perfringens type A vaccine, was the first multi-toxin vaccine to reach the market in 2021 in the US and Canada. Currently only available in Canada, the US, and South Africa, the vaccine has been shown to be effective against NE caused by Clostridium perfringens type A in broiler chickens.
Vaccine composition
The vaccine utilizes a recombinant attenuated Salmonella vector, or RASV, which is a way of using a non-disease-causing bacterium to get the necessary antigenic information to the birds’ immune system. This is a well-studied technology that has been in use since the 1980s.
A piece of DNA (plasmid) that holds the codes for fragments of the two most important toxins produced by Clostridium perfringens (C. perfringens), specifically the alpha and the netB toxins, are incorporated into the Salmonella vector. When the vaccine is introduced into the chicken’s gut, it stimulates the development of mucosal antibodies against the alpha and netB toxin. This means that the majority of antibodies will also be created right at the intestinal cell lining where the protection against NE is most needed, the intestinal mucosa. The vaccine vector is designed in such a way that it can replicate for only a short period of time before dying off while not causing disease.
Vaccine efficacy
Huvepharma® has indicated that data on vaccine efficacy has been collected both in experimental trials and on Canadian and US commercial farms, with promising results.
During experimental trials in the US in preparation of the US and Canadian registration dossiers, an infection challenge model was used where birds were challenged with Eimeria maxima and C. perfringens and given either no vaccine, a placebo vaccine, or the Clostridium perfringens type A vaccine. Three separate trials were done with these treatment groups and found:
For the on-farm trials in the US, Huvepharma® grouped all data together from all farms and data was collected from the winter through the summer 2021. This included all different production types (conventional, No Antibiotics Ever (NAE), organic), as well as farms with and without NE history. The sample represented over 11 million birds given the vaccine at day-of-age in the hatchery, and over 18 million birds not given the vaccine. Compared to the birds that didn’t receive the Clostridium perfringens type A vaccine, vaccinated birds on average had higher livability scores, higher weights and a lower FCR. The table below shows averages across many different on-farm situations and there can be variability in individual farms, depending on the history of NE, nutrition, anticoccidial program, etc.
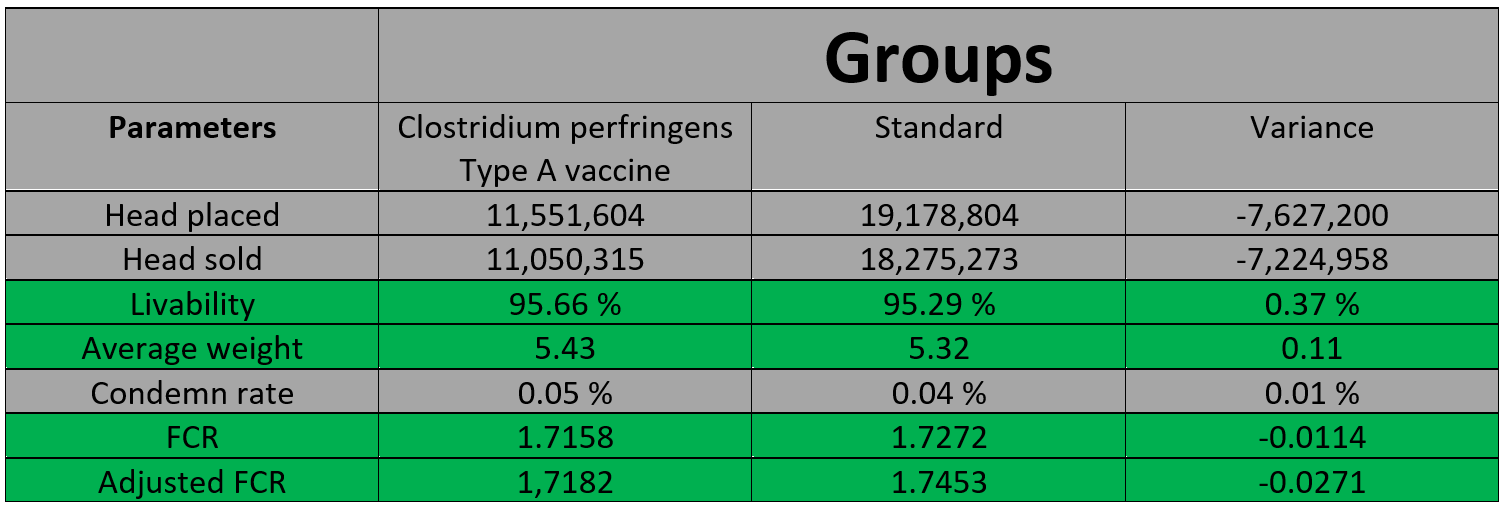
Huvepharma®’s farm studies are on-going in Canada and as an example, results have been presented from a raised-without-antibiotics (RWA) farm in Ontario which had no significant NE challenges in the past. The study included 3 control barns (vaccinated for Marek’s, bronchitis, and coccidiosis) and 4 test barns (received the control vaccines plus the Clostridium perfringens Type A vaccine), and chicks were placed in December 2021. For every control barn there was a test barn, and an extra test barn on another location was added. Average results across all barns showed that in test barns livability increased 1.8-2.6%, final weights were between 7 and 10g higher, FCR was 2-points lower (1.58 versus 1.60), and condemns were between 0.05 and 0.07% higher than in control barns.
These demonstrated performance improvements in a barn without previous NE challenges would not typically be expected with a preventive vaccine and underscores the presence and impacts of subclinical NE and the ability of this vaccine to potentially mitigate those impacts.
In a subsequent trial in Ontario between November 2021 and October 2022 with both RWA and conventional flocks, non-significant improvements were seen in birds that received the Clostridium perfringens Type A vaccine, with nearly 3 points lower FCR (1.607 compared to 1.636), and 97g heavier market weights when controlling for days to market.
An additional field trial in Ontario between December 2021 and January 2023 looked at the impact of the vaccine on RWA flocks both with and without antibiotic alternatives in feed. Compared to flocks with in-feed antibiotic alternatives and no vaccine, flocks with no alternatives plus the vaccine had 21g heavier market weights when controlling for days to market, poorer feed conversion (1.622 versus 1.558), and 1.4% lower mortality.
In the West, trials took place in Alberta and British Columbia on RWA Ross 308/708 mixed-sex flocks. Results across all barns showed that compared to controls, in test barns:
Practical use
Because this vaccine uses a Salmonella vector, it cannot be administered in flocks given antibiotics simultaneously, as antibiotics would potentially interfere with the Salmonella vector. The vaccine is recommended to be administered by coarse spray at the hatchery on day old chicks and has a 21-day withdrawal period. The vaccine is safe to be mixed with and administered at the same time as bronchitis and coccidiosis vaccines. The Clostridium perfringens type A vaccine is being sold directly to veterinarians and the majority of hatcheries across Canada have accessed this vaccine and have administered it as requested by veterinarians and farmers.
Further background info about necrotic enteritis
What causes necrotic enteritis?
Necrotic enteritis is caused by a bacteria called Clostridium perfringens (C. perfringens), derived from the Latin for “burst through”. C. perfringens exists everywhere in our environment and humans have lived with this bacterium for millions of years. However, when predisposing factors are present, C. perfringens can cause negative effects in many animal species, including broiler chickens.
C. Perfringens likes to live in the gut because it prefers an environment without oxygen (anaerobic). It is a normal inhabitant of the intestines of healthy chickens. However, when there are other factors present that cause some changes or damage to the gut – for example, coccidiosis, specific dietary compositions, a slower feed passage through the intestine, or anything that promotes bacterial imbalance in the gut – this can allow C. perfringens to flourish and produce toxins. It is actually these toxins, not the bacterium itself, that leads to NE and the associated performance losses and mortality.
The two main toxins involved in NE are the alpha and netB toxin. The alpha toxin is reported to be found in all C. perfringens strains and is an enzyme that acts on the fat layer of cells by cutting open cell membranes in a suggested calcium-dependent manner. The NetB toxin creates pores in cell membranes, which creates ruptures in the gut lining and thus allows important molecules to be lost. It is in these ways that the two toxins cause the intestinal damage that is ubiquitous with NE.
Impacts of Necrotic enteritis
Clinical illness due to NE is usually short, with the only signs being a severe depression followed by a sudden increase in flock mortality, up to 50% in some cases (Timbermont et al., 2011).
The subclinical form of NE causes the greatest economic losses in the poultry industry because the disease goes undetected and could remain unprevented or untreated. Subclinical NE is characterized by intestinal mucosal damage with no overt clinical signs, but which leads to production losses due to poor digestion and absorption, reduced weight gain, and increased feed conversion ratio (Timbermont et al., 2011). Liver lesions are also found during inspection at slaughter and can increase the number of condemnations.
Controlling Necrotic enteritis
There are three basic needs for NE control:
Other tools for controlling NE include:
References
Hargis, B.M. 2016. Overview of necrotic enteritis in poultry. Merck Veterinary Manual. Available online, https://www.merckvetmanual.com/poultry/necrotic-enteritis/overview-of-necrotic-enteritis-in-poultry
De Keyser, K. 2022. Vaccination against necrotic enteritis due to Clostridium perfringens Type A. Webinar to the BC Poultry Symposium. Available online, https://bcpoultrysymposium.com/videos/
Timbermont, L., Haesebrouck, F., Ducatelle, R., and Van Immerseel, F., 2011. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathology, 40, 341-347. Available online, https://www.tandfonline.com/doi/full/10.1080/03079457.2011.590967
Tsiouris, V. 2016. Poultry management: a useful tool for the control of necrotic enteritis in poultry. Avian Pathology, 45, 323-325. Available online, https://www.tandfonline.com/doi/full/10.1080/03079457.2016.1154502.