


 Français
Français


The Canadian Food Inspection Agency is changing the withdrawal time for certain poultry vaccines which will allow for more flexibility of their use. The information below describes the vaccines involved, the implementation timeline and what information farmers should look for to record vaccine withdrawals on the Flock Information Reporting Form.
Background
The current withdrawal period in Canada for all vaccines used in food producing animals is a minimum of 21 days, regardless of the type of vaccine or species in which they are used. The withdrawal period is product-specific and determined based on the review of data regarding additives found within it.
The CFIA-CCVB evaluated a proposal by the poultry sector to reduce the withdrawal period for certain poultry vaccines manufactured in the United States (U.S.) from 21 days to 7 days. This change is intended to accommodate Canadian broiler chicken production practices, which differ from those in the U.S., and would allow for a vaccine booster closer to slaughter age. The CCVB collaborated with the Canadian Association of Poultry Veterinarians to develop a shortlist of vaccines and worked with subject-matter experts to compile scientific evidence supporting scenarios where a 7-day withdrawal period could be considered.
Considering all the vaccines on the list are manufactured in the U.S., the CCVB also worked with the United States Department of Agriculture’s Center for Veterinary Biologics (USDA-CVB) to ensure that this change would not create any regulatory challenges.
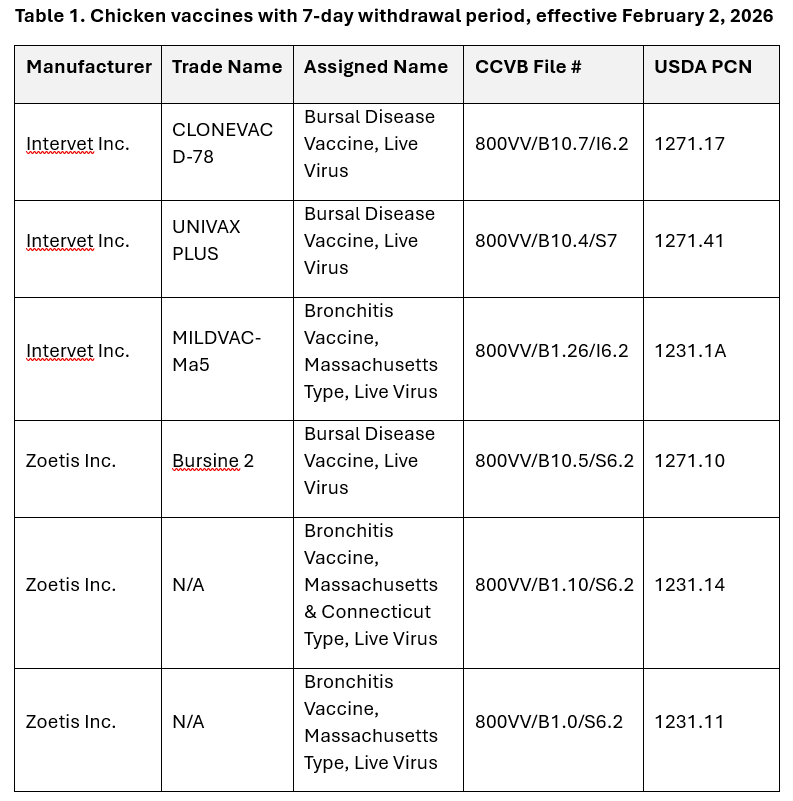
Results/Next steps
Export Only labelling, indicating this reduced withdrawal period, has been approved by both the USDA-CVB and the CCVB for use on these products in Canada. As per their new labels, the products listed in Table 1 below will state a 7-day withdrawal period.
The implementation date for the change to a 7-day withdrawal period when using these vaccines in Canada is set for February 2, 2026. As such, for a given product, a short transition period is expected; where both product labelled with a 21-day withdrawal period and that with a 7-day withdrawal period, may circulate simultaneously in the market immediately after the set implementation date. However, after the February 2, 2026 implementation date, all products listed in Table 1, regardless of the product label, will have a 7-day withdrawal.
Flock Information Reporting Form
This notice uniquely applies to the six vaccines listed in Table 1; withdrawal times for other vaccines have not changed. The withdrawal period stated on the respective label for any other vaccines must continue to be followed.
Farmers should continue to follow the instructions on the veterinary prescription for any vaccines prescribed to their flocks and report those withdrawal periods accordingly on the Flock Information Reporting Form.
Version 9 of the Flock Information Reporting Form must be used as of January 1, 2026.

